For each of the following compounds (here shown in their acidic forms), write the form that predominates in a solution with a pH = 5.5:
e. +NH4 (pKa = 9.4)
f. HC≡N (pKa = 9.1)
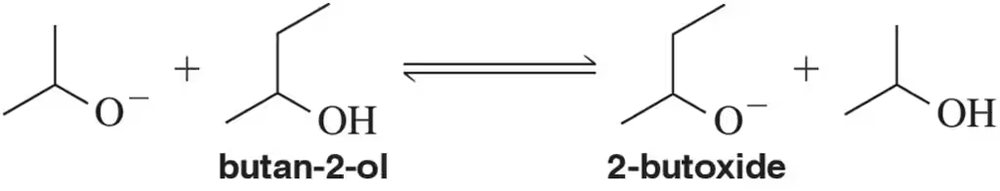
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:46m
1:46mMaster Why we use pKa instead of pH. with a bite sized video explanation from Johnny
Start learning