a. Explain why 1-bromo-2,2-dimethylpropane has difficulty undergoing both SN2 and SN1 reactions.
b. Can it undergo E2 and E1 reactions?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: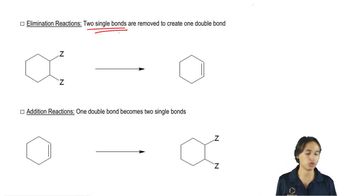


 9:36m
9:36mMaster Drawing the E2 Mechanism. with a bite sized video explanation from Johnny
Start learning