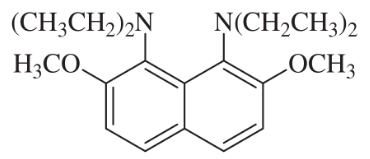
The following drugs are synthesized using the methods in this chapter and in previous chapters. Devise a synthesis for each, starting with any compounds containing no more than six carbon atoms.
(a) Phenacetin, used with aspirin and caffeine in pain-relief medications.