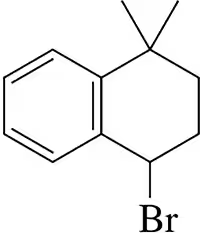
A halogenation intended to make compound A formed B instead.
(d) Without looking it up, would you expect C–Ha or C–Hb to have the lower bond-dissociation energy?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 6:14m
6:14mMaster Side-Chain Halogenations with a bite sized video explanation from Johnny
Start learning