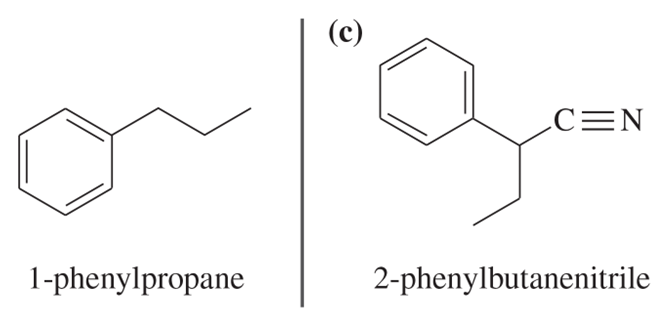
(c) Based on what you know about the relative stabilities of alkyl radicals and benzylic radicals, predict the product of addition of HBr to 1-phenylpropene in the presence of a free-radical initiator.
(d) Propose a mechanism for this reaction.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 6:14m
6:14mMaster Side-Chain Halogenations with a bite sized video explanation from Johnny
Start learning