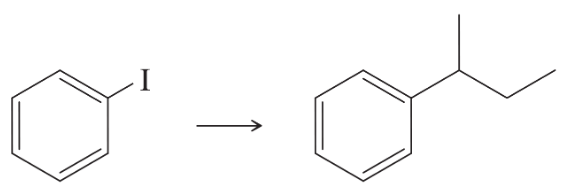
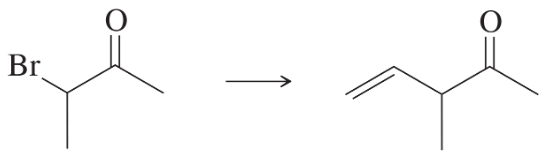
Develop syntheses for the following compounds. As starting materials, you may use cyclopentanol, alcohols containing no more than four carbon atoms, and any common reagents and solvents.
(b) 1-chloro-1-ethylcyclopentane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 0:24m
0:24mMaster Intro to Predict the Product with a bite sized video explanation from Johnny
Start learning