To this point, hydrogenation has always been an exothermic process. Using the numbers from Figure 21.6, calculate ∆Hohydr for each step of the reduction of benzene.
∆H1 + ∆H2 + ∆H3 = ∆Htot = ―49.5 kcal /mol (―208 kJ/mol)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: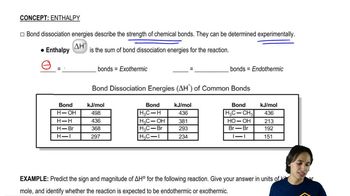


 4:09m
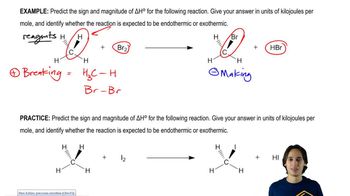
4:09mMaster How to calculate enthalpy using bond dissociation energies. with a bite sized video explanation from Johnny
Start learning