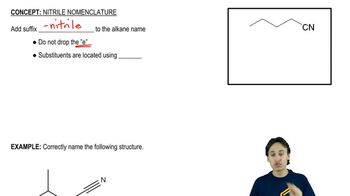
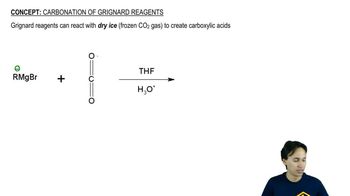
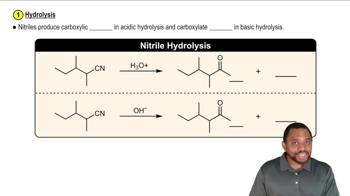
(a) Outline the syntheses indicated in Solved Problem 18-2, beginning with aldehydes and alkyl halides.
(b) Both of these syntheses of 1-phenylbuta-1,3-diene form the central double bond. Show how you would synthesize this target molecule by forming the terminal double bond.