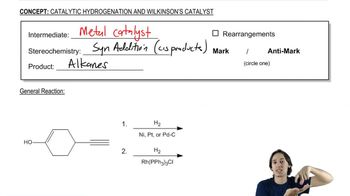
Draw the products of the following reactions. If the products can exist as stereoisomers show what stereoisomers are formed.
j. (E)-2,3-dichloro-2-butene + H2, Pd/C
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: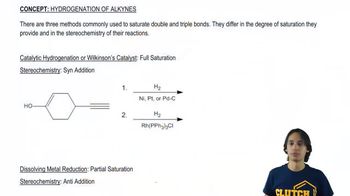


 5:21m
5:21mMaster General properties of catalytic hydrogenation. with a bite sized video explanation from Johnny
Start learning