4-Hydroxy- and 5-hydroxyaldehydes exist primarily as cyclic hemiacetals. Draw the structure of the cyclic hemiacetal formed by each of the following:
d. 4-hydroxyheptanal
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: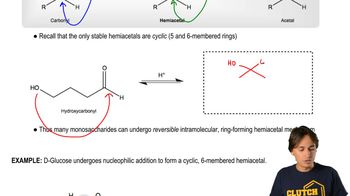

 6:23m
6:23mMaster Monosaccharides - Forming Cyclic Hemiacetals with a bite sized video explanation from Johnny
Start learning