Textbook Question
Suggest a reagent and a reactant that could be combined to make each of the following alcohols.
(d)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: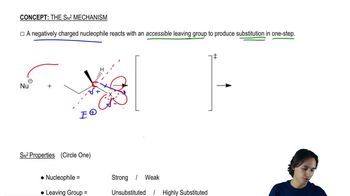

 2:26m
2:26mMaster Forming alcohols through Oxymercuration-Reduction. with a bite sized video explanation from Johnny
Start learning