Thiols can be prepared from the reaction of thiourea with an alkyl halide, followed by hydroxide-ion-promoted hydrolysis.
a. Propose a mechanism for the reaction.
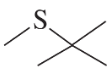
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: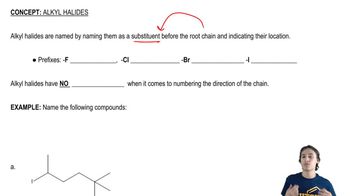
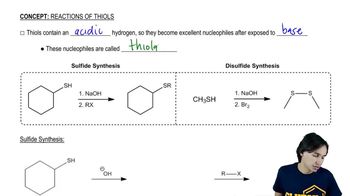
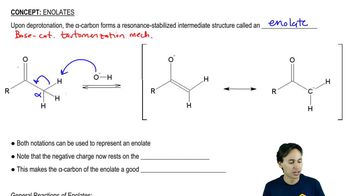

 3:21m
3:21mMaster The mechanism of Sulfide Synthesis. with a bite sized video explanation from Johnny
Start learning