What product is formed when 1-bromopropane reacts with each of the following nucleophiles?
c. CH3S−
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: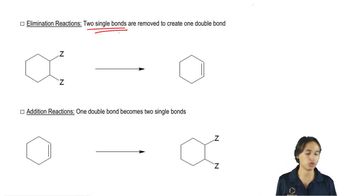



 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning