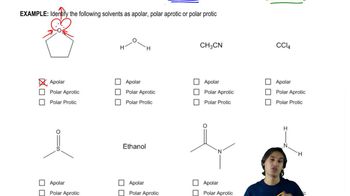
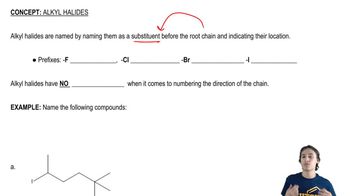
[FIGURE: KEY MECHANISM 7-1]
Show what happens in step 2 of the example if the solvent acts as a nucleophile (forming a bond to carbon) rather than as a base (removing a proton).
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: