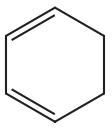
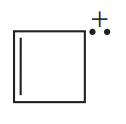
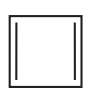
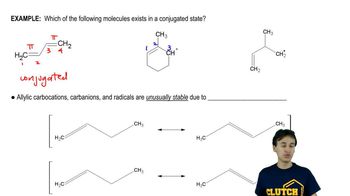
(i) Classify the following molecules as aromatic, nonaromatic, or antiaromatic.
(ii) For the aromatic and antiaromatic molecules, solve for n in Hückel’s/Breslow’s rule. For the other molecules, explain which of the rules of aromaticity is being broken.
(d)