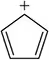
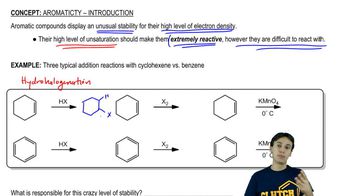
Biphenyl has the following structure.
c. The heat of hydrogenation for biphenyl is about 418 kJ/mol (100 kcal/mol). Calculate the resonance energy of biphenyl.
d. Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain the difference in the resonance energies of naphthalene and biphenyl.