How many signals are produced by each of the following compounds in its
b. 13C NMR spectrum?
1.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
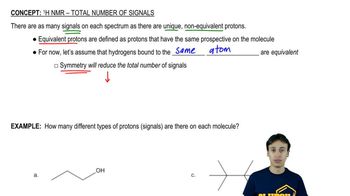
Verified video answer for a similar problem: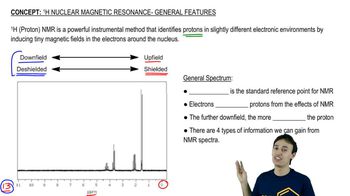


 4:m
4:mMaster 13C NMR General Features with a bite sized video explanation from Johnny
Start learning