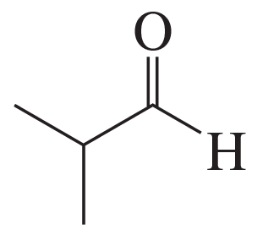
How can 1,2-, 1,3-, and 1,4-dinitrobenzene be distinguished by
b. 13C NMR spectroscopy?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: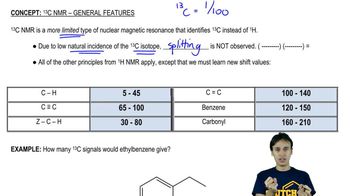

 4:m
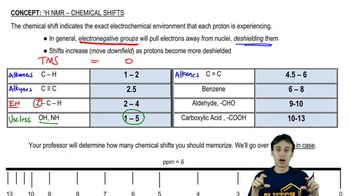
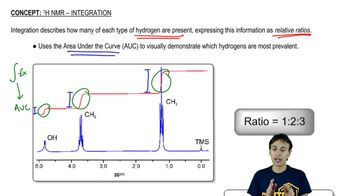
4:mMaster 13C NMR General Features with a bite sized video explanation from Johnny
Start learning