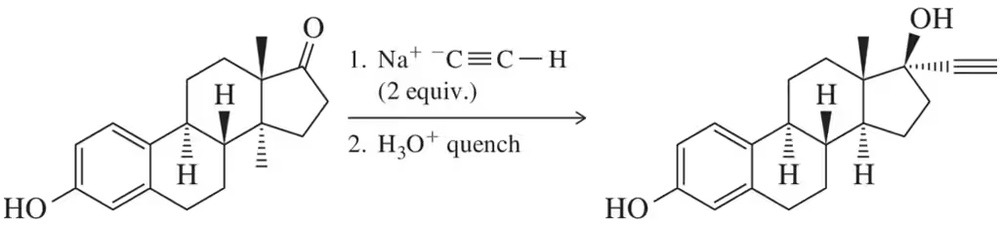
Show how you might synthesize the following compounds, using acetylene and any suitable alkyl halides as your starting materials. If the compound given cannot be synthesized by this method, explain why.
a. hex-1-yne
b. hex-2-yne
c. hex-3-yne
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:19m
4:19mMaster Understanding how to convert terminal alkynes to alkynides. with a bite sized video explanation from Johnny
Start learning