Give two sets of reactants (each set including an alkyl halide and a nucleophile) that could be used to synthesize the following alkyne:
CH3CH2C≡CCH2CH2CH2CH3
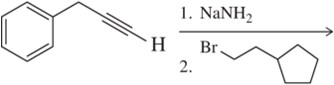
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: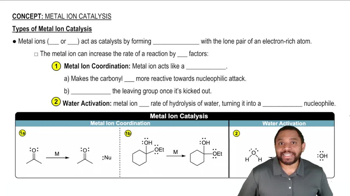
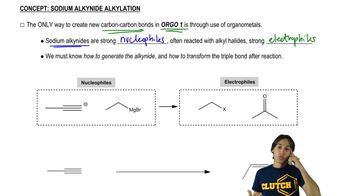

 4:19m
4:19mMaster Understanding how to convert terminal alkynes to alkynides. with a bite sized video explanation from Johnny
Start learning