(i) Using the periodic trend, choose the more electronegative atom in each pair. For one pair, you'll need to look at the actual Pauling values. (ii) For which one? (iii) Why?
(d) B vs. Si
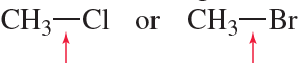
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 11:33m
11:33mMaster Differences between ionic, polar and covalent bonds with a bite sized video explanation from Johnny
Start learning