Predict the product for the following substitution reactions. Indicate whether each reaction likely proceeds by an SN1 or SN2 mechanism.
(a)
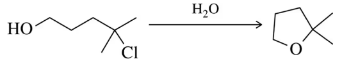
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:32m
3:32mMaster How do we predict if the mechanism is SN1 or SN2? with a bite sized video explanation from Johnny
Start learning