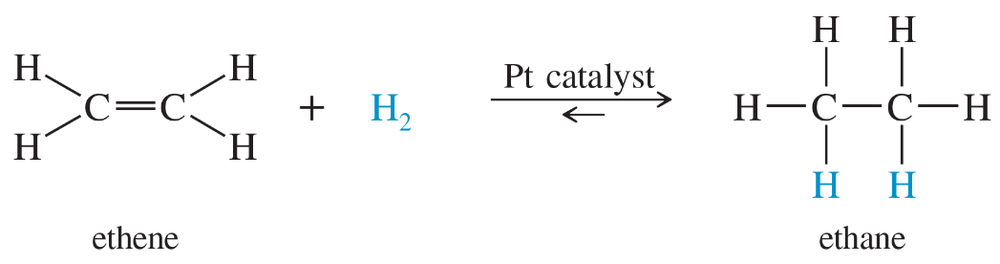
Calculate ∆G°, ∆H°, and ∆S° for the following acid–base reactions. Rationalize the value of ∆H° based on the structure of the conjugate bases. [Assume T = 298 K.]
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: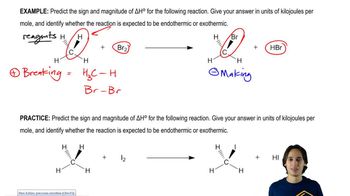

 2:46m
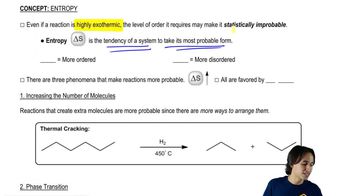
2:46mMaster Explaining what entropy is. with a bite sized video explanation from Johnny
Start learning