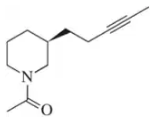
Drawing on what you know about the stereochemistry of alkene addition reactions,
b. predict the configuration of the product of the reaction.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:15m
2:15mMaster Double halogenation of alkynes. with a bite sized video explanation from Johnny
Start learning