Predict the products you would get when the following alkenes react under the following conditions: (i) H2SO4, H2O and (ii) 1. Hg(OAc)2, H2O , 2. NaBH4
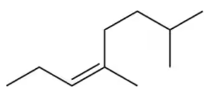
(d)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:15m
2:15mMaster Double halogenation of alkynes. with a bite sized video explanation from Johnny
Start learning