Multiple Choice
Name the alcohol that was used to create the following urethane molecule.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: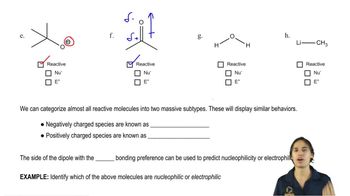


 1:51m
1:51mMaster Step-Growth Polymers: Urethane Concept 1 with a bite sized video explanation from Johnny
Start learning