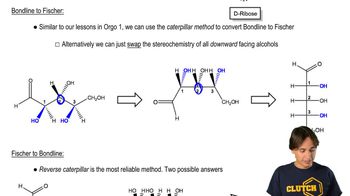
Multiple Choice
How many stereoisomers are possible for a 2-ketohexose, considering D and L isomerism?
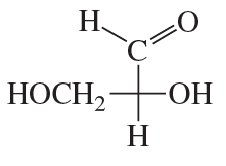
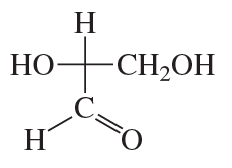
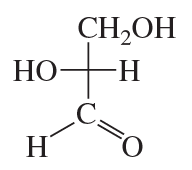
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:47m
6:47mMaster Monosaccharides - D and L Isomerism with a bite sized video explanation from Johnny
Start learning