Calculate the equilibrium constant for the acid–base reaction between the reactants in each of the following pairs:
c. CH3NH2 + H2O
d. CH3N+H3 + H2O
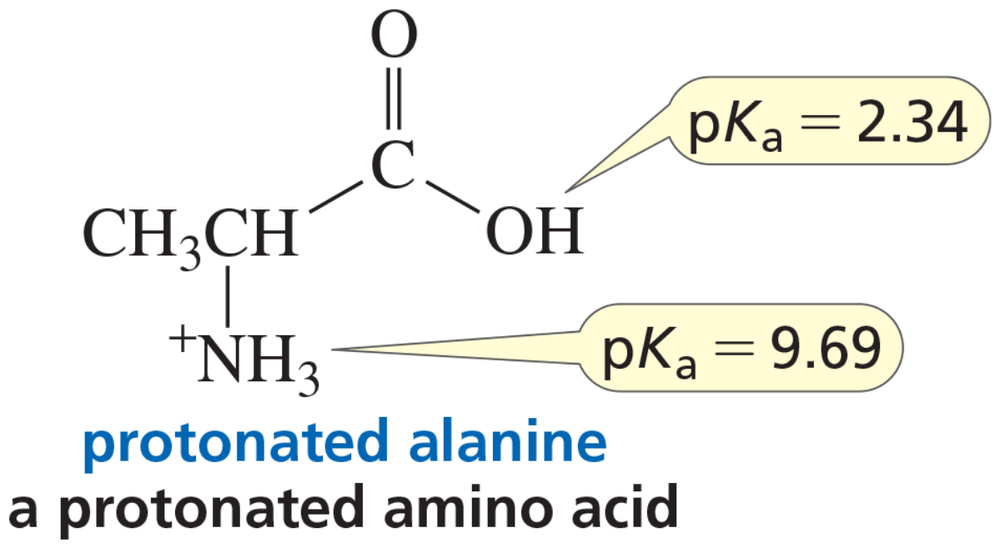
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:46m
1:46mMaster Why we use pKa instead of pH. with a bite sized video explanation from Johnny
Start learning