A naturally occurring amino acid such as alanine has a group that is a carboxylic acid and a group that is a protonated amine. The pKa values of the two groups are shown.
c. Draw the structure of alanine in a solution at physiological pH (pH 7.4).
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: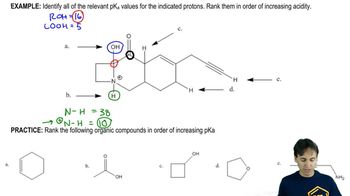

 1:46m
1:46mMaster Why we use pKa instead of pH. with a bite sized video explanation from Johnny
Start learning