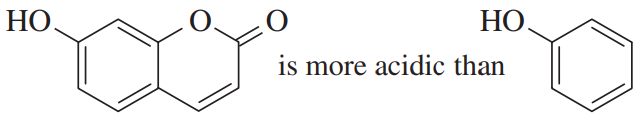The pKa of ascorbic acid (vitamin C, page 55) is 4.17, showing that it is slightly more acidic than acetic acid (CH3COOH, pKa 4.74).
a. Show the four different conjugate bases that would be formed by deprotonation of the four different OH groups in ascorbic acid.
b. Compare the stabilities of these four conjugate bases, and predict which OH group of ascorbic acid is the most acidic.