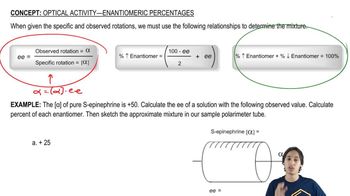
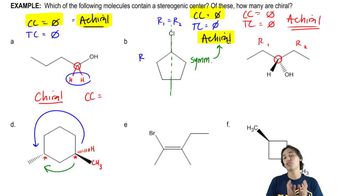
Textbook Question
(i) Which of the following pairs of compounds would you expect to have different physical properties?
(ii) What is the relationship between each of the pairs?
(iii) Assign the absolute configuration of each stereocenter to confirm your answer.
(c)