For each reaction, give the expected substitution product, and predict whether the mechanism will be predominantly first order (SN1) or second order (SN2).
c. 1-iodo-1-methylcyclohexane + ethanol
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: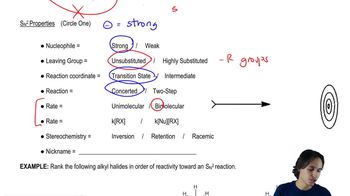


 3:32m
3:32mMaster How do we predict if the mechanism is SN1 or SN2? with a bite sized video explanation from Johnny
Start learning