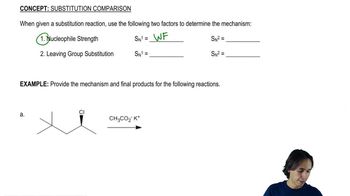
a. Optically active 2-bromobutane undergoes racemization on treatment with a solution of KBr. Give a mechanism for this racemization.
b. In contrast, optically active butan-2-ol does not racemize on treatment with a solution of KOH. Explain why a reaction like that in part (a) does not occur.