Draw the product of each of the following sigmatropic rearrangements:
d.
![Chemical structure showing a [1,3] sigmatropic rearrangement with products labeled A and B.](https://static.studychannel-dev.pearsondev.tech/courses/organic-chemistry/thumbnails/7d32594d-bf7d-4007-b4a3-ad32290b6c43)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: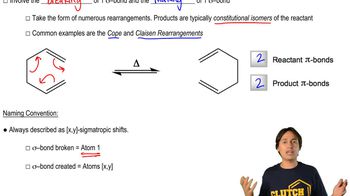


 3:51m
3:51mMaster Definition of Sigmatropic Shifts with a bite sized video explanation from Johnny
Start learning