Show how you would accomplish the following synthetic conversions.
(b) 1-bromo-2-phenylethane → 3-phenylpropan-1-amine
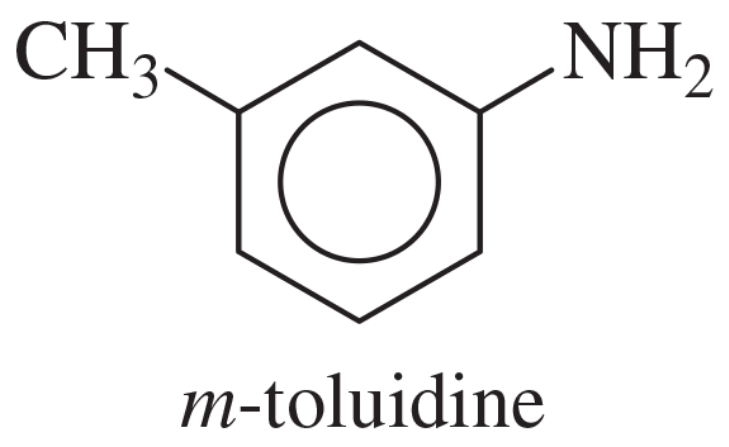
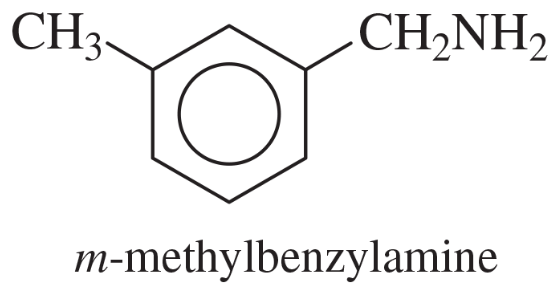
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: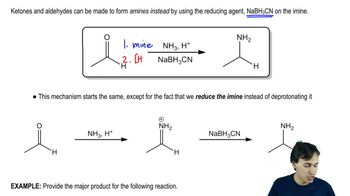



 9:12m
9:12mMaster The Primary Amines Flowchart with a bite sized video explanation from Johnny
Start learning