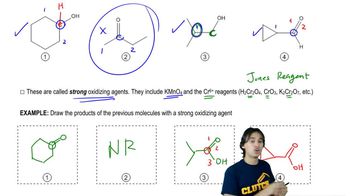
(a) Based on Figure 24.23, explain why meta-dihydroxybenzene is not oxidized to meta-quinone.
(b) If a meta-quinone is not produced, what would you expect the product of the oxidation of meta-dihydroxybenzene to be?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: