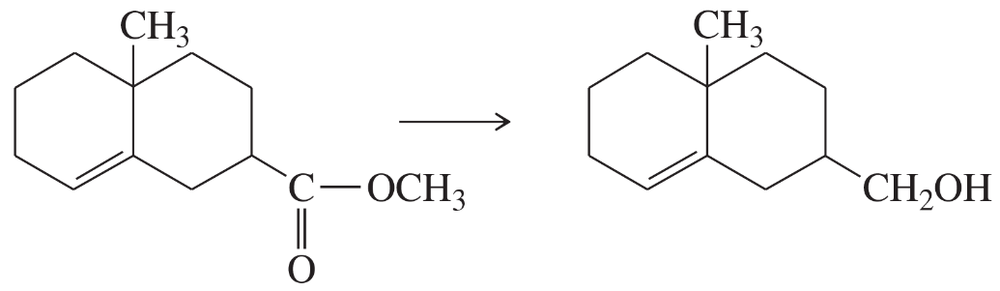
Suggest the most appropriate method for each of the following laboratory syntheses. In each case, suggest both a chromium reagent and a chromium-free reagent.
(f) 1-methylcyclohexanol → 2-methylcyclohexanone (several steps)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

