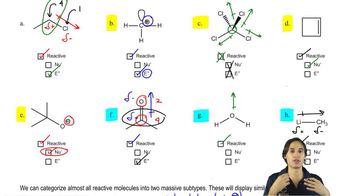
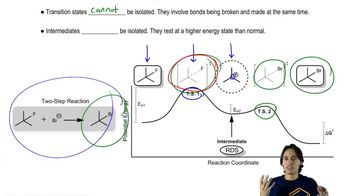
Both NaBH4 and NaBD4 are commercially available, and D2O is common and inexpensive. Show how you would synthesize the following labeled compounds, starting with butan-2-one.
(a)
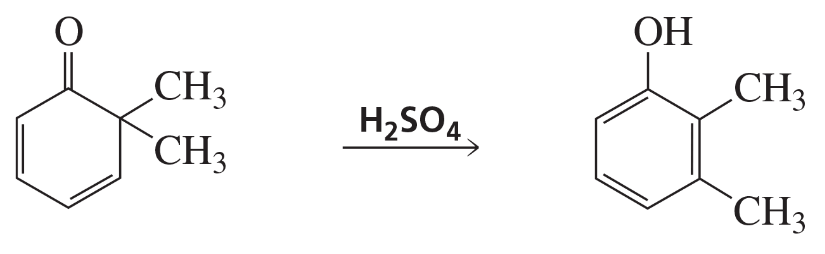
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: