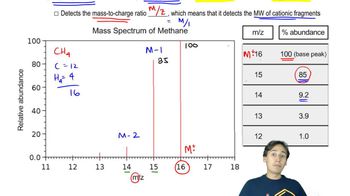
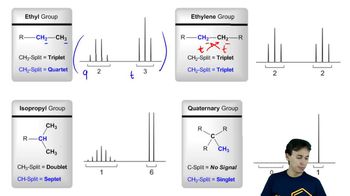
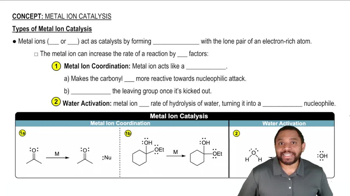
Propose the number of carbons for a compound that exhibits the following peak in its mass spectrum:
(M)+• at m/z = 72, relative height = 38.3% of base peak
(M+1)+• at m/z = 73, relative height = 1.7% of base peak
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: