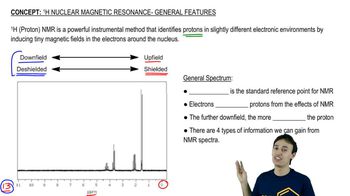
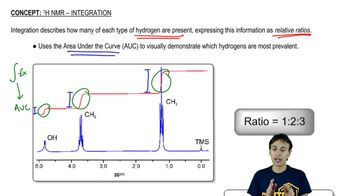
The following proton NMR spectrum is of a compound of molecular formula C3H8O.
<IMAGE>
(a) Propose a structure for this compound.
(b) Assign peaks to show which protons give rise to which signals in the spectrum.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: