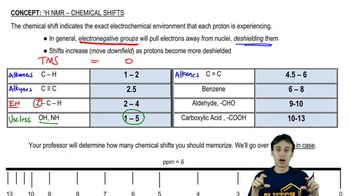
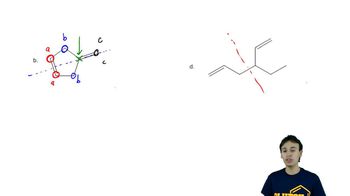
Determine the number of different kinds of protons in each compound.
(a) 1-chloropropane
(b) 2-chloropropane
(c) 2,2-dimethylbutane
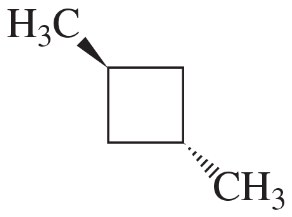
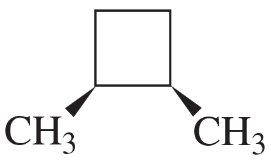
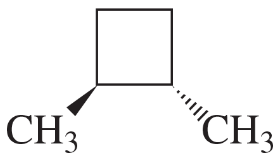
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 4:35m
4:35mMaster General Assumption for 1H NMR Signals with a bite sized video explanation from Johnny
Start learning