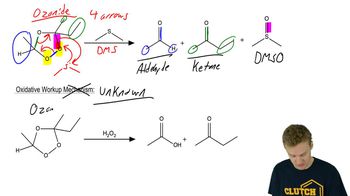
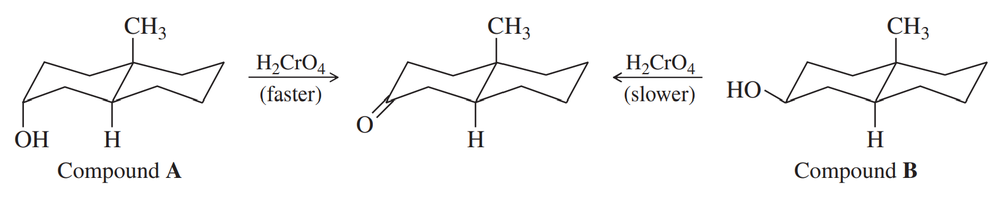
Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
a. 1-methylcyclopentene
b. 2-methylcyclopentyl tosylate
c. 2-methylcyclopentanone
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: