a. How could you use IR spectroscopy to determine whether the following reaction had occurred?
b. After purifying the product, how could you determine whether all the NH2NH2 had been removed?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: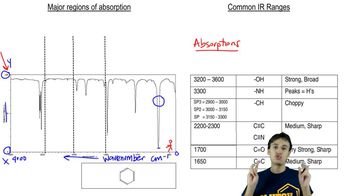

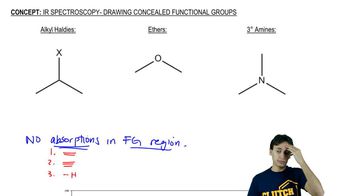

 16:47m
16:47mMaster Common IR Frequencies with a bite sized video explanation from Johnny
Start learning