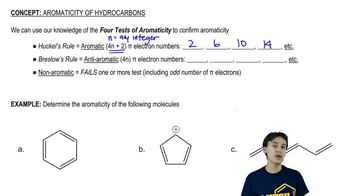
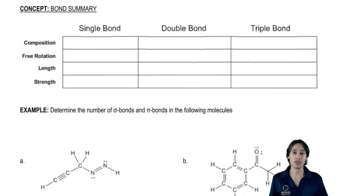
Give five examples of structures with this formula (C6H12). At least one should contain a ring, and at least one should contain a double bond.
Hint: If you prefer to use a formula, elements of unsaturation = 1/2(2C + 2 - H)
C = number of carbons
H = number of hydrogens