Predict the product(s) that would result when molecules (a)–(p) are allowed to react under the following conditions: (v) 1. TsCl, Et₃N 2. NaOt-Bu (vi) H₂SO₄ If no reaction occurs, write 'no reaction.'
(o)
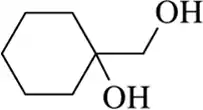
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 0:38m
0:38mMaster Intro to Substitution/Elimination Problems with a bite sized video explanation from Johnny
Start learning