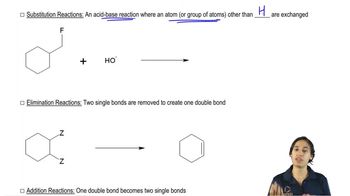
Predict the product of the following substitution/addition reactions involving phenoxides. [Because this problem represents a review of current and previous material, section numbers have been provided for your reference.]
(d)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:58m
0:58mMaster Remembering general patterns of reactions. with a bite sized video explanation from Johnny
Start learning