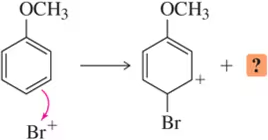
We discuss the following reactions in subsequent chapters. Given the mechanisms shown, draw the mechanism of the reverse reaction.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 5:14m
5:14mMaster How to tell if a molecule will be reactive or not. with a bite sized video explanation from Johnny
Start learning