Identify the following reactions as oxidation or reduction (based on what happens to the organic molecule).
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: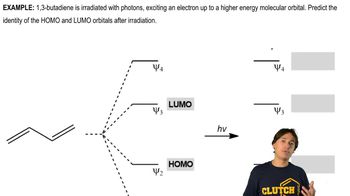


 6:02m
6:02mMaster General Features of Redox with a bite sized video explanation from Johnny
Start learning