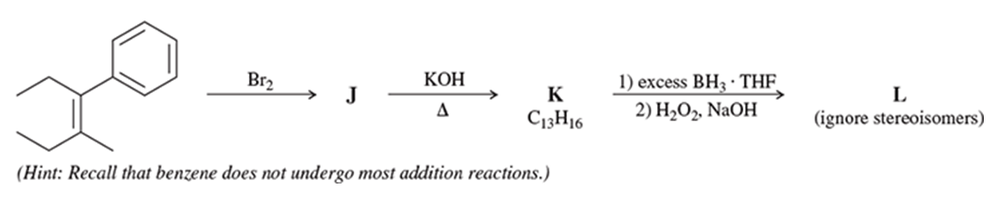
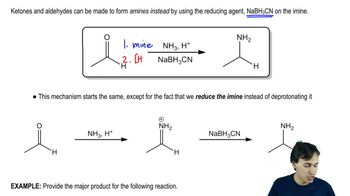
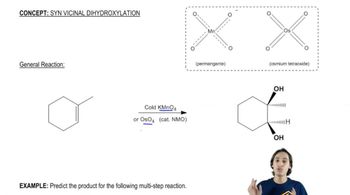
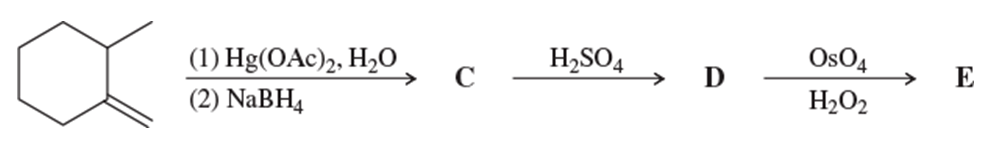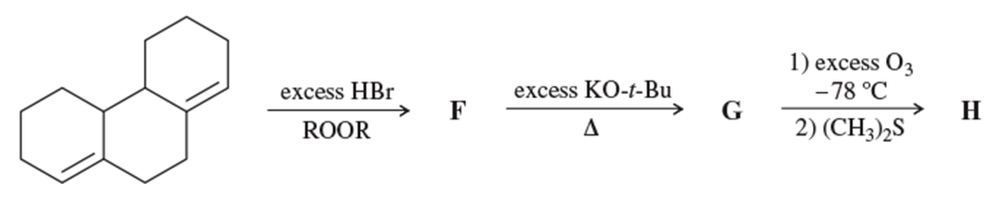Using hex-1-ene as your starting material, show how you would synthesize the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)
d. hex-2-yne
e. hexan-2-one
f. hexanal