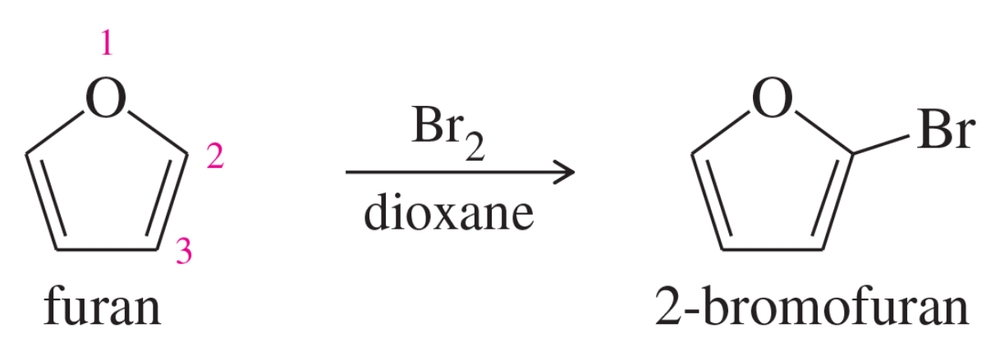
Biphenyl is two benzene rings joined by a single bond. The site of substitution for a biphenyl is determined by (1) which phenyl ring is more activated (or less deactivated), and (2) which position on that ring is most reactive, using the fact that a phenyl substituent is activating and ortho, para-directing.
a. Use resonance forms of a sigma complex to show why a phenyl substituent should be ortho, para-directing.
(vi)